Bioreactors - The heartbeat of Biopharmaceutical Manufacturing For many biopharmaceutical manufacturing companies, the bioreactor is…

2023 Breakdown: FDA 483 Warning Letters
What’s the Word?
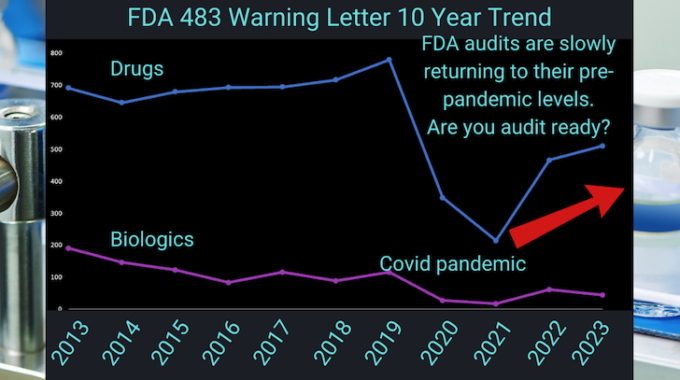
In 2023, the FDA issued a total of five hundred and ten (510) 483 Warning Letters in the Drug Category. In the Biologics category, forty-five (45) 483 Warning Letters were issued.
FDA 483 Warning Letters are issued following a site visit when conditions found do not meet FDA’s stringent regulations.
A deeper analysis shows an interesting trend in the words most commonly used in the FDA 483 Warning Letters issued in 2023.
Let’s take a look at each category separately: Drug and Biologics.
Category: Drug
In the Drug Category, there were 3 words that were the most commonly used in the 483 Warning Letters issued in 2023:
- Records
- Procedures
- Batch
A word cloud is a useful tool that helps easily visualize the top 3 words that appear in the Warning Letters in comparison to other words. Figure 1 below depicts the most commonly used words. The larger the word appears in the word cloud, the more commonly that word appears in the Warning Letters.

Figure 1: A word cloud depicting the most commonly used words in FDA 483 Warning Letters issued in 2023 in the Drug category.
Citations and Top Words – Drug Category
In the Drug category, the word “records” appeared a total of 70 times in 2023.
An example of a citation issued using the word “record” is shown below:
“Appropriate controls are not exercised over computers or related systems to assure that changes in master production and control records or other records are instituted only by authorized personnel.”
The word “procedures” appeared a total of 66 times in 2023.
An example of a citation issued using the word “procedures” is shown below:
“Your firm failed to establish [adequate] written procedures for production and process controls designed to assure that the drug products have the identity, strength, purity, and quality that they are purported or represented to possess.”
The word “batch” appears a total of 57 times in 2023.
An example of a citation issued using the word “batch” is shown below:
“Written records of investigations into [unexplained discrepancies] [the failure of a batch or any of its components to meet specifications] do not [always] include the conclusions and follow-up.”
Category: Biologics
In the Biologics Category, there were 3 words that were the most commonly used in the 483 Warning Letters issued in 2023:
- Blood
- Donor
- Procedure
The word cloud for the Biologics category is shown in Figure 2 below. The larger the word appears in the word cloud, the more commonly that word appears in the Warning Letters.

Figure 2: A word cloud depicting the most commonly used words in FDA 483 Warning Letters issued in 2023 in the Biologics category.
Citations and Top Words – Biologics Category
In the Biologics category, the word “blood” appeared a total of 18 times in 2023.
An example of a citation issued using the word “blood” is shown below:
“Records are not concurrently maintained with the performance of each significant step in the [collection] [processing] [compatibility testing] [storage] [distribution] of each unit of blood and blood components so that all steps can be clearly traced.”
The word “donor” appeared a total of 12 times in 2023.
An example of a citation issued using the word “donor” is shown below:
“Failure to maintain [donor] [processing] [storage and distribution] [compatibility testing] [quality control] [general] records.”
The word “procedure” appears a total of 10 times in 2023.
An example of a citation issued using the word “procedure” is shown below:
“The standard operating procedure fails to include a written description of schedules and procedures for equipment maintenance and calibration.”
Is your facility FDA Audit Ready?
Based on this analysis, we can see that maintaining proper records, whether it be for batches, process equipment, donor information, or procedure execution, is important.
We’ve already established that the numbers of FDA 483 Warning Letters are picking back up. In light of this, here are a few questions to think about when getting – or rather, staying – ready for your next FDA audit:
- Are your manufacturing batch records clearly executed?
- Are equipment records up to date?
- Are all personnel sufficiently trained on procedures?
- Are the records for blood collection generated at the same time it’s being collected?
- Are all donor records properly maintained?
It’s important to keep a close eye on what keeps coming up in these Warning Letters. These 483 Warning Letters can help you better understand what the FDA is keeping their pulse on during inspections and site visits, so you can stay ready for your next audit.