Bioreactors - The heartbeat of Biopharmaceutical Manufacturing For many biopharmaceutical manufacturing companies, the bioreactor is…
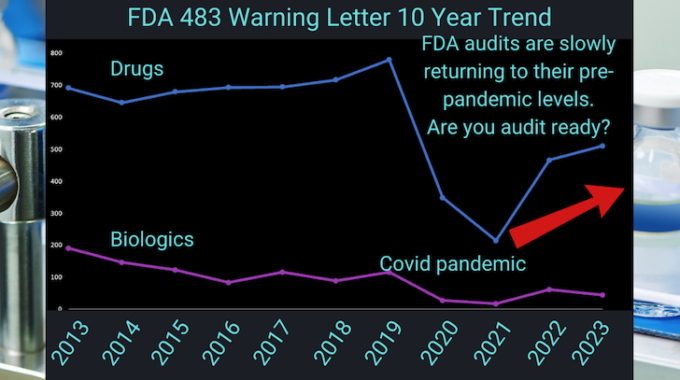
Are you FDA audit ready?
A look back at the last decade in the numbers of FDA 483 Warning Letters issued show a significant dip during the height of the pandemic.
FDA 483 Warning Letters are issued following a site visit when conditions found do not meet FDA’s stringent regulations.
The numbers of FDA 483 Warning Letters being issued have started to rebound.
A couple of questions to think about:
Are all critical equipment and instrument calibrations up to date?
Are your training and onboarding processes effective?
Are deviations addressed in a timely manner?
Are your SOPs clear and up to date?
Are effective CAPAs in place?
Now might be a good time to get your house in order.
Your next FDA audit is most likely to be sooner rather than later.
As they say, stay ready so you don’t have to get ready.